We sit in between the huge astronomical distances and masses and the sub-atomic scale of the most elementary particles of matter.
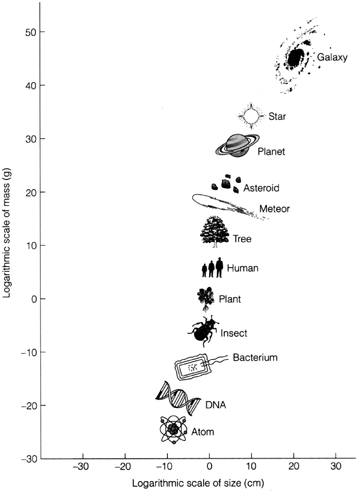
Figure 2.1 The mass and size ranges of some important ingredients of the Universe. Our choice of centimetres and grams as units places us close to the centre of things.
Despite the introduction of universal metric standards by international commissions and government ministers, the ordinary worker took little notice of edicts about units, especially in Britain where a huge multiplicity of special units were in play throughout every branch of industry and commerce. By the middle of the nineteenth century, the industrial revolution had created diverse human sub-cultures of engineers and brewers, accountants and metalworkers, timekeepers and ship workers, all of whom needed ways of measuring the materials that they managed and manipulated. The result was an explosion of units of measure. Every type of material began to have its own standard of strength and tolerance, quantity and weight. Not only were these units anthropocentric they were profession-centric as well. Brewers liked one choice of volume measure, water engineers another; jewellers measured weight differently to sailors and architects. When I was a child there was a common brand of lined exercise book that would be used for making notes at school. They always had red or blue covers and the outside back cover of the book listed all the peculiar Imperial measures of length, area, capacity and weight (see Figure 2.2).
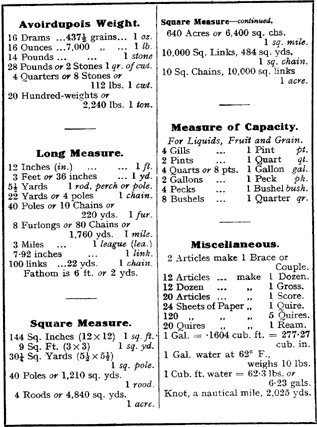
Figure 2.2 A typical set of miscellaneous weights and measures from an English self-help book of the 1950s.17
For the engineer and the practical person of affairs this was convenient, useful and no doubt very profitable. But for anyone seeking an integrated natural philosophy it made human knowledge appear fragmented and idiosyncratic. A visitor from another planet would be perplexed by the need for different measures of weight when buying gold, apples or sealing wax.
MAINTAINING UNIVERSAL STANDARDS
‘There was a crooked man who built a crooked house.’
Nursery rhyme
By the second half of the nineteenth century, engineers, industrialists and scientists were becoming overwhelmed by the profusion of ad hoc units and measures. The industrial revolution had accelerated the development of every imaginable industry. Manufacturing, machining, measuring, designing, building – these were the rages of the age and they spawned more and more units.
Within the halls of science the existence of standard lengths and masses was not entirely satisfactory for the purist either. Every time standard masses were handled with their special tongs their mass would be very slightly changed. It would vary slightly as atoms were evaporated from their surfaces or dust deposited from the atmosphere. They were not really constant.18 Nor were they universal. Suppose that a signal had been received from an engineer on another planet asking us how big we were. It would be no use sending an answer in metres or kilograms and then responding to the inevitable reply, ‘What are they?’ by telling our extraterrestrial correspondent that they were objects kept in glass containers in Paris. Unfortunately the quest for universal standards had created examples which were neither standard nor universal.
Within science the driving force for rationalisation came from the study of electricity and magnetism. Different systems of units were in use by different groups of scientists and had different relationships with the traditional metric units for mass, length, time and temperature.
The first general response to these problems came from Lord Rayleigh and James Clerk Maxwell. In his Presidential address to the British Association for the Advancement of Science in 1870 Maxwell advocated the introduction of standards which are not tied to special objects, like standard metres19 or kilograms kept in special conditions. For standards like these can never really be constant. The standard mass in Paris will lose and gain molecules all the time. Measures of time that are defined, like the day, by the rotation of the Earth or, like the year, by its orbit of the Sun likewise cannot be constant. As the rotation of the Earth slows, and our solar circuit changes, so these standards will very slowly drift. They may be defined in extrahuman terms but they are not candidates for ultimate standards. Maxwell had spent a good deal of time studying the behaviour of molecules in gases and was very impressed by the way in which each molecule of hydrogen was the same as all the others. This was quite different to dealing with large, everyday objects where every one was different. Maxwell saw an opportunity to use the sameness of molecules to define standards absolutely:
‘Yet, after all, the dimensions of our earth and its time of rotation, though, relatively to our present means of comparison, very permanent, are not so by any physical necessity. The earth might contract by cooling, or it might be enlarged by a layer of meteorites falling on it, or its rate of revolution might slowly slacken, and yet it would continue to be as much a planet as before.
But a molecule, say of hydrogen, if either its mass or its time of vibration were to be altered in the least, would no longer be a molecule of hydrogen.
If, then, we wish to obtain standards of length, time, and mass which shall be absolutely permanent, we must seek them not in the dimensions, or the motion, or the mass of our planet, but in the wave-length, the period of vibration, and the absolute mass of these imperishable and unalterable and perfectly similar molecules [i.e.
1 comment